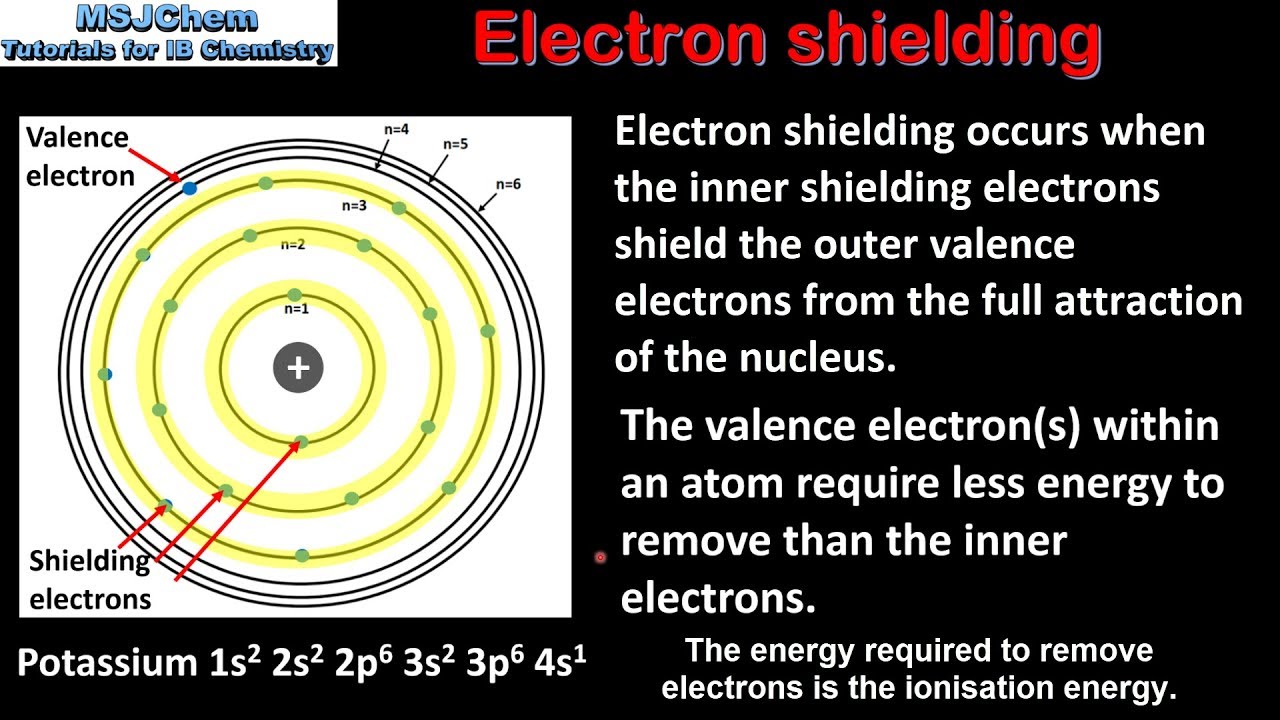
Electron shielding is a fundamental concept in chemistry and physics that plays a crucial role in understanding atomic structure and behavior. It refers to the phenomenon where the electrons in an atom can shield the outer electrons from the full charge of the nucleus. This effect influences various properties of atoms, including their size, ionization energy, and reactivity.
The study of electron shielding is essential for anyone looking to grasp the complexities of chemical bonding and atomic interactions. By understanding how inner electrons affect the outer shell electrons, we can better predict the behavior of elements in different chemical reactions. In this article, we will delve into the definition of electron shielding, its significance in atomic theory, and its implications in various applications.
Whether you are a student, a professional in the field, or just someone curious about the intricacies of atomic structure, this comprehensive guide will provide you with a wealth of knowledge. We will explore the key aspects of electron shielding, supported by data and references from credible sources, to ensure a thorough understanding of this important topic.
Table of Contents
- 1. Definition of Electron Shielding
- 2. Importance of Electron Shielding
- 3. Factors Affecting Electron Shielding
- 4. Applications of Electron Shielding
- 5. Examples of Electron Shielding in Action
- 6. Conclusion
- 7. References
1. Definition of Electron Shielding
Electron shielding, also known as electron screening, occurs when inner-shell electrons repel outer-shell electrons, resulting in a reduction of the effective nuclear charge experienced by those outer electrons. This phenomenon is crucial for understanding the structure of atoms and how they interact with one another.
1.1 The Concept of Effective Nuclear Charge
The effective nuclear charge (Zeff) is the net positive charge experienced by an electron in a multi-electron atom. It takes into account the actual nuclear charge (Z) and the extent of shielding provided by the inner electrons. The formula for effective nuclear charge can be expressed as:
Zeff = Z - S
Where S is the shielding constant, representing the extent to which inner electrons shield the outer electrons from the nucleus.
2. Importance of Electron Shielding
Understanding electron shielding is vital for several reasons:
- It helps explain trends in atomic size across the periodic table.
- It influences the ionization energy of elements.
- It plays a significant role in chemical bonding and molecular geometry.
2.1 Trends in Atomic Size
As you move down a group in the periodic table, the number of electron shells increases, leading to greater electron shielding. This causes the outermost electrons to be further from the nucleus, resulting in larger atomic radii.
3. Factors Affecting Electron Shielding
Several factors can influence the effectiveness of electron shielding:
- Number of Inner Electrons: More inner electrons generally provide better shielding.
- Electron Configuration: The arrangement of electrons in various orbitals can affect shielding.
- Distance from the Nucleus: Electrons further from the nucleus are more shielded than those close to it.
3.1 Electron Configuration and Shielding
The arrangement of electrons in an atom, known as electron configuration, can significantly impact how well the inner electrons shield the outer electrons. For instance, in transition metals, the presence of d-electrons can affect the effective nuclear charge experienced by the outer s-electrons.
4. Applications of Electron Shielding
Electron shielding has numerous applications across various fields:
- Chemistry: Understanding chemical reactivity and bonding.
- Physics: Predicting atomic behavior in different states of matter.
- Material Science: Developing materials with specific electrical properties.
4.1 Chemical Reactivity
In chemistry, electron shielding can help predict how atoms will react with one another. For example, elements with similar electron shielding properties often exhibit similar chemical behaviors.
5. Examples of Electron Shielding in Action
Several examples illustrate the concept of electron shielding:
- Group Trends: The increasing atomic size and decreasing ionization energy down a group are primarily due to enhanced electron shielding.
- Ion Formation: Understanding how atoms lose or gain electrons to form ions is greatly informed by the concept of electron shielding.
5.1 Case Study: Sodium (Na) and Chlorine (Cl)
Sodium (Na) and chlorine (Cl) provide a classic example of electron shielding. Sodium has one outer electron that experiences significant shielding from its inner electrons. Conversely, chlorine has a higher effective nuclear charge due to its greater number of protons and less effective shielding from its inner electrons, making it more electronegative.
6. Conclusion
In summary, electron shielding is a fundamental concept that helps explain various atomic properties and behaviors. By understanding how inner electrons shield outer electrons from the nucleus, we can better predict trends in atomic size, ionization energy, and chemical reactivity. The implications of electron shielding extend across disciplines, from chemistry and physics to material science.
We encourage you to leave your thoughts in the comments section below, share this article with others interested in atomic theory, and explore more of our content to deepen your understanding of related topics.
7. References
1. Atkins, P. W., & Friedman, R. (2011). Molecular Quantum Mechanics. Oxford University Press.
2. Levine, I. N. (2013). Quantum Chemistry. Pearson.
3. Zumdahl, S. S., & Zumdahl, S. A. (2014). Chemistry. Cengage Learning.
Article Recommendations
- Wallet With Pull Tab
- Brand Building_0.xml
- Arianna Lima 2024
- Healthy Habits_0.xml
- Madison Beer Nude Leak
- Data Driven_0.xml
- Financial Empowerment_0.xml
- Global Impact_0.xml
- Talulah Riley
- Efficient Strategies_0.xml
![[Term 2] Account for (b) In case of transition elements, ions of same](https://i2.wp.com/d77da31580fbc8944c00-52b01ccbcfe56047120eec75d9cb2cbd.ssl.cf6.rackcdn.com/29d92af9-fbe4-4169-b199-a7823a0465e3/shielding-effect-----teachoo.jpg)
