When delving into the fascinating world of chemistry, understanding the molecular structure of compounds is pivotal. One such compound, CH3Cl, also known as methyl chloride, presents an interesting case study due to its unique Lewis structure and molecular geometry. By comprehensively exploring the CH3Cl Lewis structure, we can gain valuable insights into the arrangement of its atoms, the type of bonds formed, and how these factors influence its chemical properties and reactivity patterns. This article aims to provide a detailed overview of CH3Cl, shedding light on its Lewis structure and molecular geometry.
The Lewis structure of a molecule illustrates the arrangement of electrons among atoms, providing a visual representation of how atoms bond together. In the case of CH3Cl, understanding this structure is critical for interpreting its molecular geometry and the angles between its bonds. Furthermore, the molecular geometry of CH3Cl plays a crucial role in its behavior and interactions in various chemical reactions. By examining both the Lewis structure and molecular geometry, we can better comprehend the characteristics of methyl chloride and its applications in different fields.
As we embark on this exploration of CH3Cl, we will answer essential questions regarding its Lewis structure and molecular geometry. From understanding the significance of valence electrons to the impact of molecular shape on properties, this article will serve as a comprehensive guide for anyone interested in the structural intricacies of methyl chloride.
What is the Lewis Structure of CH3Cl?
The Lewis structure of CH3Cl can be determined by following specific steps that involve counting valence electrons and arranging them to satisfy the octet rule. Methyl chloride consists of one carbon atom, three hydrogen atoms, and one chlorine atom. Here’s how to construct the Lewis structure:
- **Count the total number of valence electrons**:
- Carbon (C): 4 valence electrons
- Hydrogen (H): 1 valence electron (3 H atoms = 3)
- Chlorine (Cl): 7 valence electrons
- **Arrange the atoms**: Carbon is the central atom, with three hydrogen atoms and one chlorine atom bonded to it.
- **Distribute the electrons**: Place a single bond (2 electrons) between the carbon and each hydrogen and between carbon and chlorine.
- **Check for octet completion**: Carbon has 8 electrons around it (3 single bonds with H and 1 single bond with Cl), hydrogen has 2 electrons (satisfied), and chlorine has 8 electrons (satisfied).
This leads us to the Lewis structure of CH3Cl, which shows that carbon is bonded to three hydrogens and one chlorine through single bonds, with no lone pairs on the carbon atom.
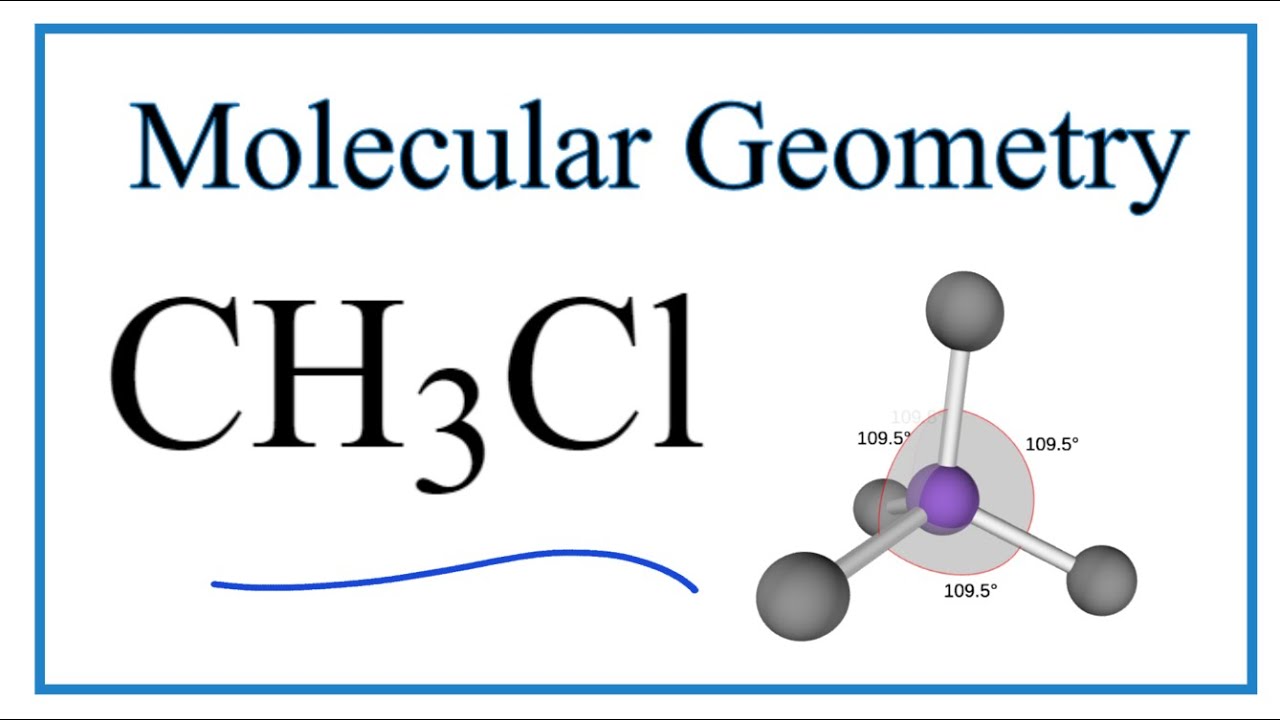
What is the Molecular Geometry of CH3Cl?
The molecular geometry of CH3Cl can be determined using the VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron pairs around a central atom will arrange themselves to minimize repulsion. In CH3Cl, we identify the following:
- **Central atom**: Carbon (C)
- **Bonding pairs**: 4 (3 with hydrogen, 1 with chlorine)
- **Lone pairs**: 0
Given that there are four bonding pairs and no lone pairs, the molecular geometry of CH3Cl is classified as tetrahedral. The bond angles in a tetrahedral structure are approximately 109.5 degrees.
How Does the Molecular Geometry of CH3Cl Affect Its Properties?
The tetrahedral shape of CH3Cl significantly influences its physical and chemical properties. The bond angles and symmetry affect factors such as polarity, boiling point, and reactivity. Here are a few implications of its molecular geometry:
- **Polarity**: Due to the presence of chlorine, which is more electronegative than carbon and hydrogen, CH3Cl is a polar molecule. This polarity affects its solubility in water and other solvents.
- **Boiling Point**: The presence of dipole-dipole interactions in polar molecules like CH3Cl results in a higher boiling point compared to non-polar compounds of similar molecular weight.
- **Reactivity**: The molecular geometry can influence how CH3Cl reacts with other chemicals, impacting its use in various applications, including as a solvent and refrigerant.
What Are the Applications of CH3Cl?
CH3Cl has several important applications across different industries, primarily due to its properties as a solvent and its role in chemical synthesis. Some of the notable applications include:
- **Solvent**: Methyl chloride is commonly used as a solvent in organic reactions and extractions due to its ability to dissolve a wide range of organic compounds.
- **Refrigerant**: It has been historically utilized as a refrigerant, although its use has declined due to environmental concerns.
- **Chemical Intermediate**: CH3Cl serves as a precursor in the production of various chemicals, including agricultural pesticides and pharmaceuticals.
What Safety Precautions Should Be Taken When Handling CH3Cl?
While CH3Cl has valuable applications, it is essential to handle this compound with care due to its toxic nature. Here are some safety precautions to consider:
- **Use Personal Protective Equipment (PPE)**: Always wear gloves, goggles, and protective clothing when handling methyl chloride.
- **Work in a well-ventilated area**: To minimize inhalation risks, ensure adequate ventilation when working with CH3Cl.
- **Proper Storage**: Store methyl chloride in labeled containers in a cool, dry place away from incompatible materials.
What Are the Environmental Concerns Related to CH3Cl?
Despite its utility, CH3Cl poses environmental concerns, particularly regarding its contribution to ozone depletion. As a halogenated compound, it can release chlorine atoms into the atmosphere, which are harmful to the ozone layer. Due to these concerns, regulations have been put in place to limit the production and use of methyl chloride, encouraging the development of safer alternatives.
Conclusion: The Significance of CH3Cl Lewis Structure and Molecular Geometry
In summary, the CH3Cl Lewis structure provides vital insights into the molecular arrangement and bonding of this compound, while its tetrahedral molecular geometry has significant implications for its chemical properties and applications. Understanding the intricacies of CH3Cl not only enhances our knowledge of organic chemistry but also highlights the importance of safety and environmental considerations in chemical usage. As we continue to explore the world of chemistry, the study of compounds like methyl chloride will remain essential in developing innovative solutions and sustainable practices.
Article Recommendations
- Piper Parabo
- Career Advancement_0.xml
- Outdoor Propane Heater Table Top
- Kylie Jenner Before Surgery
- Mario Lopez
- Global Impact_0.xml
- Kamila Valieva
- Mia Hamm Soccer Player
- Chandie Yawn Nelson
- Efficient Strategies_0.xml