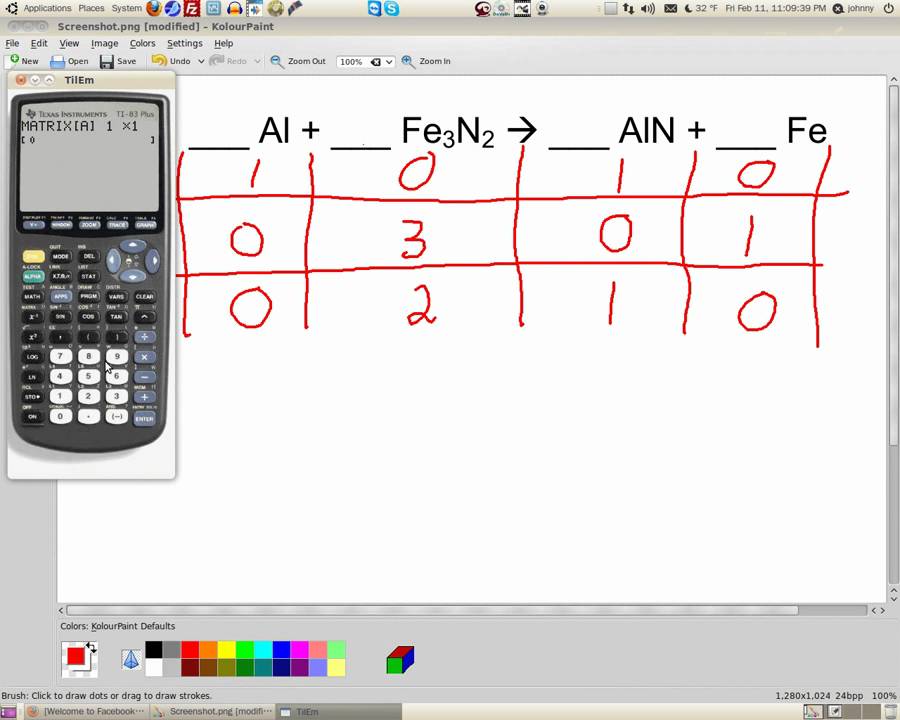
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is upheld in chemical reactions. With the increasing complexity of chemical equations, many students and professionals turn to online calculators for assistance. In this article, we will explore how to effectively use a "how to balance chemical equations calculator," the underlying principles of balancing equations, and tips to enhance your understanding of this essential topic.
Understanding how to balance chemical equations is crucial for anyone studying chemistry, whether you're a student, educator, or a professional in the field. This skill allows you to represent chemical reactions accurately and understand the quantities of reactants and products involved. As we delve deeper into this topic, we will also provide you with valuable resources, including a detailed step-by-step guide on using a calculator to balance chemical equations.
In this guide, we will cover various aspects of balancing chemical equations, from basic concepts to advanced techniques. By the end of this article, you will have a clear understanding of how to utilize a balancing chemical equations calculator effectively, along with the knowledge to tackle even the most complex equations with confidence.
Table of Contents
- What Are Chemical Equations?
- Importance of Balancing Equations
- How to Balance Chemical Equations
- Introduction to Balancing Chemical Equations Calculators
- How to Use a Balancing Chemical Equations Calculator
- Benefits of Using a Calculator
- Challenges in Balancing Equations
- Conclusion
What Are Chemical Equations?
Chemical equations represent the transformation of reactants into products during a chemical reaction. They consist of two main parts: the reactants (substances that undergo change) and the products (substances formed as a result of the reaction). For instance, in the equation:
2H2 + O2 → 2H2O
The reactants are hydrogen (H2) and oxygen (O2), while the product is water (H2O). Each substance is represented by its chemical formula, and coefficients before the formulas indicate the number of molecules involved.
Importance of Balancing Equations
Balancing chemical equations is essential for several reasons:
- Conservation of Mass: It adheres to the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
- Stoichiometry: Balancing equations allows for accurate calculations of reactants and products, which is crucial for stoichiometric calculations in chemistry.
- Predicting Reaction Outcomes: A balanced equation provides insights into the ratios of reactants and products, helping chemists predict the outcome of reactions.
How to Balance Chemical Equations
Balancing chemical equations can be challenging, but with practice and the right approach, it becomes more manageable. Here is a step-by-step guide to balancing chemical equations:
Step-by-Step Guide
- Write the unbalanced equation.
- Count the number of atoms for each element on both sides of the equation.
- Add coefficients to balance the atoms of each element, starting with the most complex molecule.
- Repeat the process until all elements are balanced.
- Check your work by recounting the atoms on both sides.
Common Techniques
There are several techniques that can simplify the balancing process:
- Inspection Method: Adjust coefficients by inspection to achieve balance.
- Algebraic Method: Use algebra to set up equations based on the number of atoms of each element.
- Oxidation-Reduction Method: Balance redox reactions by balancing the oxidation states.
Introduction to Balancing Chemical Equations Calculators
As chemical equations become more complex, many learners turn to balancing chemical equations calculators for assistance. These online tools simplify the balancing process, making it easier to achieve accurate results without extensive calculations. A typical calculator allows users to input an unbalanced equation, and it will return the balanced equation within seconds.
How to Use a Balancing Chemical Equations Calculator
Using a balancing chemical equations calculator is straightforward. Here’s a simple guide:
- Open your preferred calculator tool.
- Input the unbalanced chemical equation in the designated field.
- Click on the "Balance" button.
- Review the balanced equation provided by the calculator.
For example, if you enter:
H2 + O2 → H2O
The calculator will output the balanced equation:
2H2 + O2 → 2H2O
Benefits of Using a Calculator
Using a balancing chemical equations calculator offers several advantages:
- Time-Saving: Quickly balance complex equations without manual calculations.
- Accuracy: Reduces the risk of human error in balancing equations.
- Learning Tool: Provides instant feedback, helping learners understand the balancing process better.
Challenges in Balancing Equations
Despite the availability of calculators, some challenges remain in balancing equations:
- Complex Reactions: Some reactions may involve multiple products and reactants, making them difficult to balance.
- State Symbols: Understanding the physical states of reactants and products (solid, liquid, gas) can be challenging.
- Special Cases: Certain reactions, such as combustion or redox reactions, may require advanced techniques to balance properly.
Conclusion
In summary, mastering how to balance chemical equations is an essential skill in chemistry that lays the foundation for further studies in the field. Utilizing a balancing chemical equations calculator can simplify this process, making it easier to achieve accurate results. Whether you're a student seeking to improve your understanding or a professional needing assistance with complex equations, these calculators can be invaluable resources.
We encourage you to practice balancing chemical equations using both traditional methods and calculators. If you have any questions or would like to share your experiences, feel free to leave a comment below. Don't forget to explore our other articles for more insights into the fascinating world of chemistry!
Thank you for reading, and we hope to see you back here soon for more educational content!
Article Recommendations
- Lola Consuelos Weight Loss Ozempic
- Arianna Lima 2024
- Chandie Yawn Nelson
- Jennifer Syme Crash
- How Old Is Miguel Diaz
- Collision Repair Before And After
- How Long Do Horses Live
- Outdoor Propane Heater Table Top
- Brand Building_0.xml
- Mario Lopez