HSO3, or hydrogen sulfite, is a chemical compound that plays a crucial role in various chemical processes. It is essential in both industrial applications and laboratory settings, providing significant benefits in areas such as food preservation and environmental science. This article will explore the structure, properties, applications, and implications of HSO3, while also providing insights into its importance in everyday life.
The significance of HSO3 cannot be overstated, especially considering its wide range of applications. From acting as a preservative in the food industry to its role in various chemical reactions, HSO3 is a compound that deserves a detailed examination. In this article, we will delve deeper into the chemistry of HSO3, its uses, and its impact on the environment and health.
This comprehensive guide will cater to both chemistry enthusiasts and professionals, providing an authoritative resource on HSO3. We will also discuss safety measures and considerations when handling this compound, ensuring a trustworthy source of information.
Table of Contents
- What is HSO3?
- Chemical Structure of HSO3
- Properties of HSO3
- Applications of HSO3
- Environmental Impact of HSO3
- Health Considerations Related to HSO3
- Safety Measures for Handling HSO3
- Conclusion
What is HSO3?
Hydrogen sulfite, represented as HSO3, is an anion derived from sulfurous acid (H2SO3). It is commonly known as bisulfite and is often encountered in various forms such as sodium bisulfite (NaHSO3) or potassium bisulfite (KHSO3). HSO3 is a weak acid and a reducing agent, which makes it useful in a variety of chemical reactions.
As a reducing agent, HSO3 can donate electrons to other substances, facilitating redox reactions. This property is particularly valuable in organic chemistry, where it is utilized in the reduction of certain functional groups.
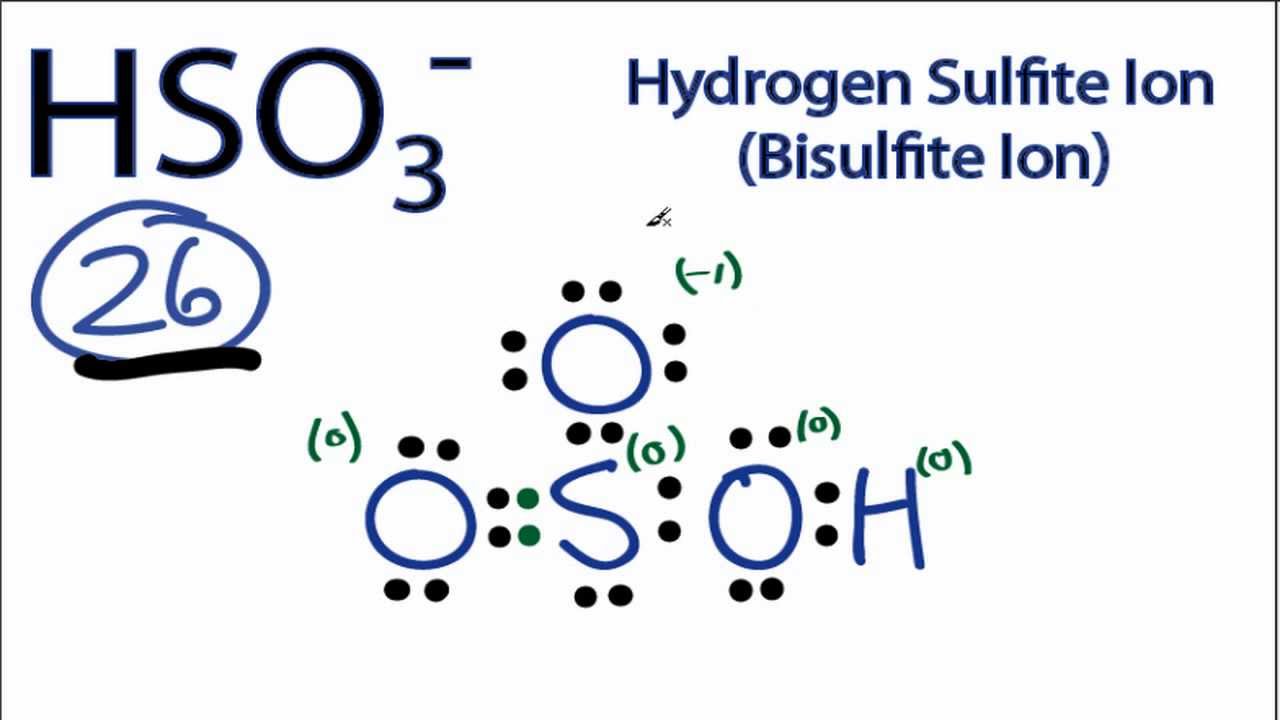
Chemical Structure of HSO3
The chemical structure of HSO3 consists of one sulfur atom bonded to three oxygen atoms and one hydrogen atom. The sulfur atom is at the center, with the hydrogen atom attached to one of the oxygen atoms, forming a bent molecular shape. The structure can be represented as follows:
- Sulfur (S): 1 atom
- Oxygen (O): 3 atoms
- Hydrogen (H): 1 atom
This arrangement gives HSO3 its unique properties and reactivity profile. The presence of the hydrogen atom allows HSO3 to act as an acid, contributing to its behavior in chemical reactions.
Properties of HSO3
HSO3 exhibits several notable properties that make it useful in various applications:
- Solubility: HSO3 is highly soluble in water, allowing it to easily participate in aqueous reactions.
- Acidity: As a weak acid, HSO3 can donate protons (H+) in solutions, affecting the pH of the environment.
- Reducing Agent: HSO3 can reduce other compounds, making it valuable in chemical synthesis.
- Stability: HSO3 is relatively stable under normal conditions, though it can decompose under extreme heat or in the presence of strong oxidizing agents.
Applications of HSO3
HSO3 has a variety of applications across different industries:
1. Food Preservation
One of the most common uses of HSO3 is as a food preservative. Sodium bisulfite, in particular, is widely used to prevent browning in fruits and vegetables, as well as to inhibit microbial growth in various food products.
2. Water Treatment
HSO3 is also employed in water treatment processes, where it helps to remove chlorine from water supplies, making it safer for consumption.
3. Textile Industry
In the textile industry, HSO3 is utilized in dyeing and bleaching processes, assisting in the removal of unwanted colors and enhancing the final appearance of fabrics.
4. Chemical Manufacturing
HSO3 is integral to various chemical manufacturing processes, serving as a reducing agent in the synthesis of organic compounds.
Environmental Impact of HSO3
The environmental impact of HSO3 primarily relates to its use in industrial processes and waste management:
- HSO3 can contribute to the formation of sulfur dioxide (SO2) when exposed to air, which is a pollutant that can lead to acid rain.
- However, when managed properly, HSO3 can help reduce harmful emissions in various industries.
Health Considerations Related to HSO3
While HSO3 is generally safe when used appropriately, there are some health considerations to be aware of:
- Some individuals may be sensitive to sulfites, leading to allergic reactions when consuming foods preserved with HSO3.
- Exposure to high concentrations of HSO3 can irritate the respiratory system, eyes, and skin.
It is essential to follow safety guidelines and regulations when handling HSO3 to minimize potential health risks.
Safety Measures for Handling HSO3
When working with HSO3, it is crucial to implement safety measures to protect oneself and the environment:
- Always wear appropriate personal protective equipment (PPE), including gloves and goggles.
- Ensure proper ventilation in workspaces to minimize inhalation of fumes.
- Store HSO3 in a cool, dry place away from incompatible substances.
- Follow all relevant safety guidelines and regulations as outlined by local authorities.
Conclusion
In summary, HSO3 is a versatile compound with significant applications across various industries, particularly in food preservation, water treatment, and chemical manufacturing. Understanding its properties, uses, and safety considerations is crucial for anyone working with or studying this compound. By promoting safe handling practices and responsible usage, we can harness the benefits of HSO3 while minimizing its potential risks.
We encourage readers to share their thoughts on HSO3 in the comments section below. If you found this article informative, please consider sharing it with others or exploring more articles on our site.
Thank you for reading, and we hope to see you again soon!
Article Recommendations
- Personal Wifi
- Data Driven_0.xml
- Cost To Extend Garage
- How Long Do Horses Live
- What Is A Mulligan
- Piper Parabo
- Corinne Foxx
- Jennifer Syme Crash
- Outdoor Propane Heater Table Top
- Three Cornered Hat Crossword Clue
![[HSO3] Bisulfite ion](https://i2.wp.com/www.chemtube3d.com/images/gallery/inorganicsjpgs/_HS03_-.jpg)
